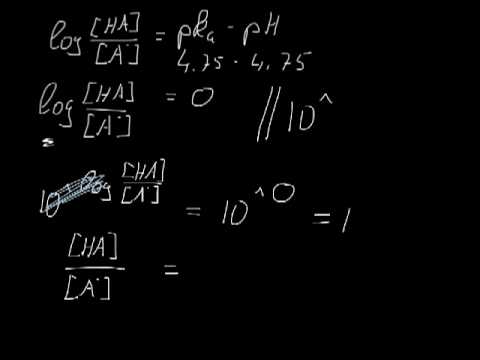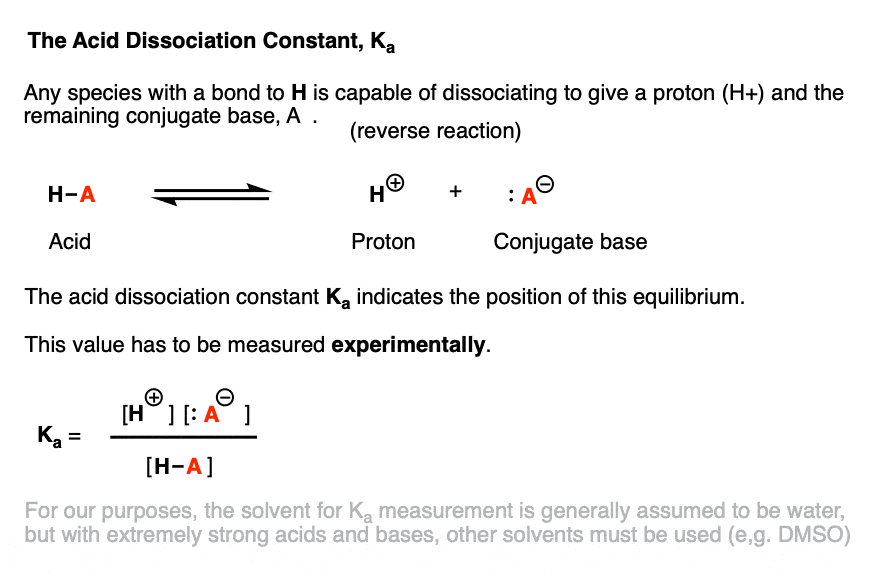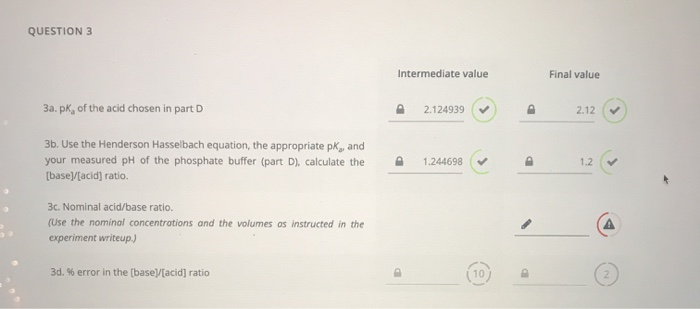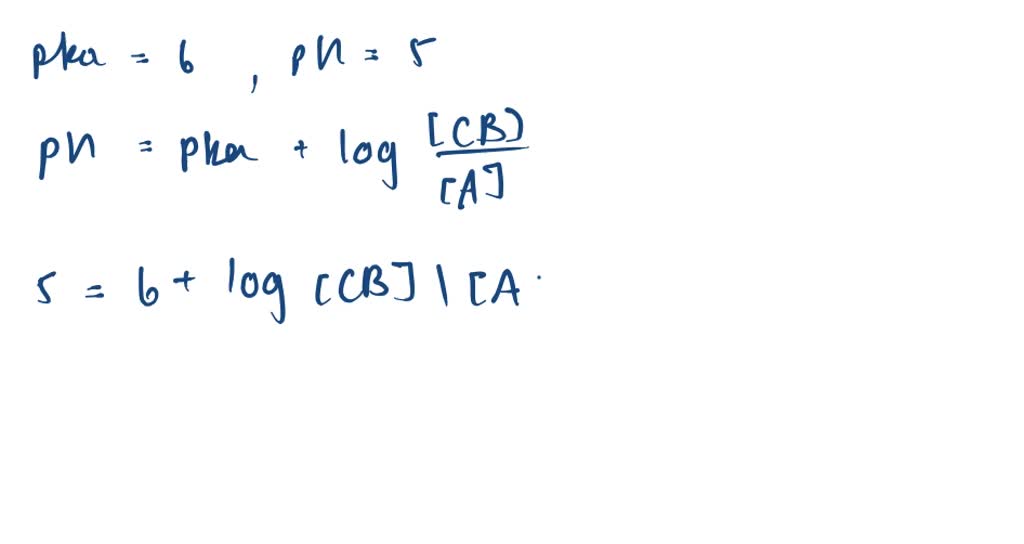
SOLVED:Calculation of Molar Ratios of Conjugate Base to Weak Acid from pH For a weak acid with a p Ka of 6.0, calculate the ratio of conjugate base to acid at a

Ionization of Acid And Bases - Arrhenius concept of Acid And Base Ionisation, Explanation, Determination ionisation constant of Acid base, Examples And FAQS
![SOLVED: Acid/Base Ratio Unanswered 1 attempt left What ratio of acid to base is needed to prepare a buffer with a pH = 3.5 using the conjugate pair HCOOH/HCOO 1(Ka 1.78x10-4J? [HCOOH]/[HcOO-] = SOLVED: Acid/Base Ratio Unanswered 1 attempt left What ratio of acid to base is needed to prepare a buffer with a pH = 3.5 using the conjugate pair HCOOH/HCOO 1(Ka 1.78x10-4J? [HCOOH]/[HcOO-] =](https://cdn.numerade.com/ask_images/3e3ccf6738d048629ccc88b39c08e146.jpg)
SOLVED: Acid/Base Ratio Unanswered 1 attempt left What ratio of acid to base is needed to prepare a buffer with a pH = 3.5 using the conjugate pair HCOOH/HCOO 1(Ka 1.78x10-4J? [HCOOH]/[HcOO-] =

Acid-Base Buffers Equation & Examples | How to Calculate pH of a Buffer - Video & Lesson Transcript | Study.com
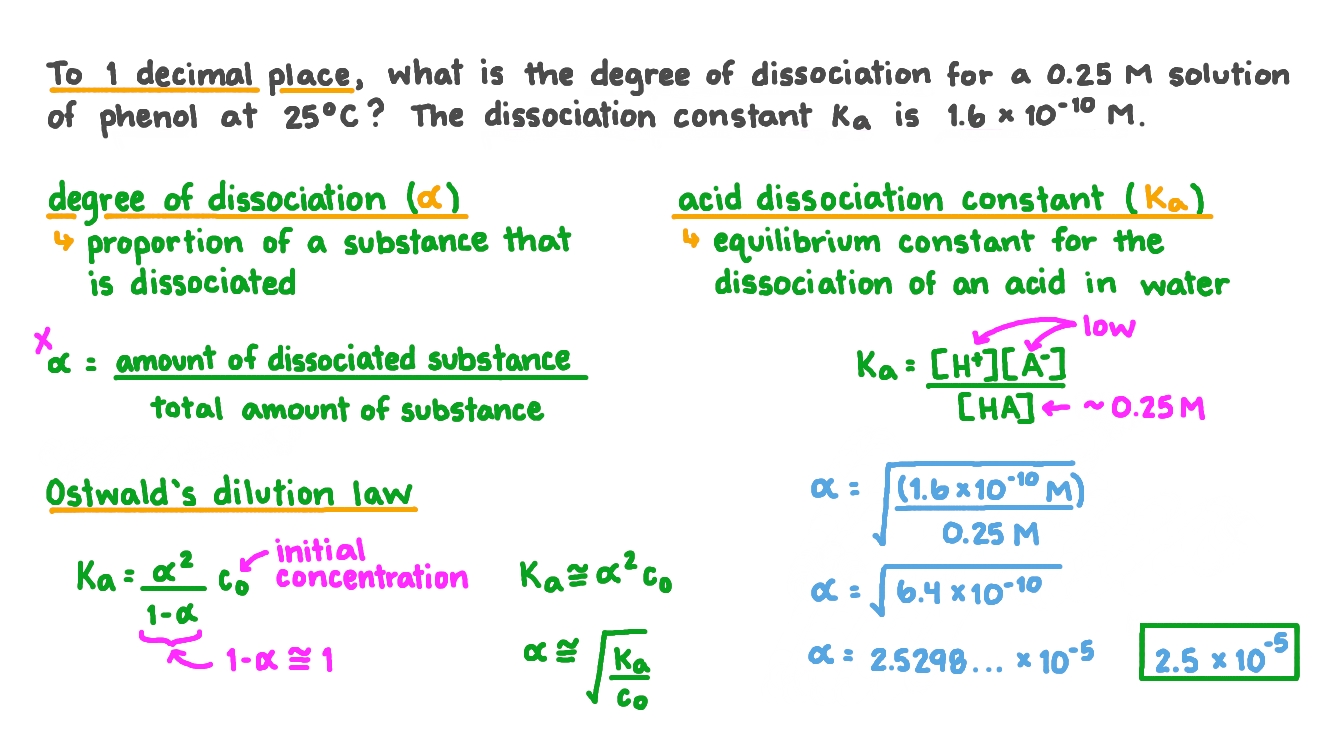
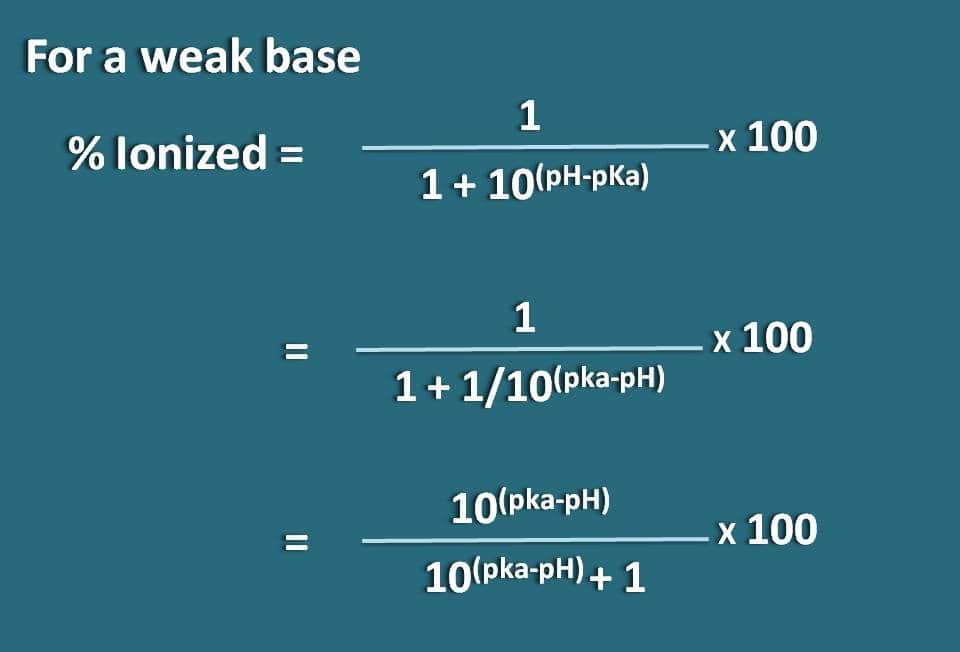
Question Video: Calculating the Degree of Dissociation of a Solution of Phenol Given the Acid Dissociation Constant | Nagwa
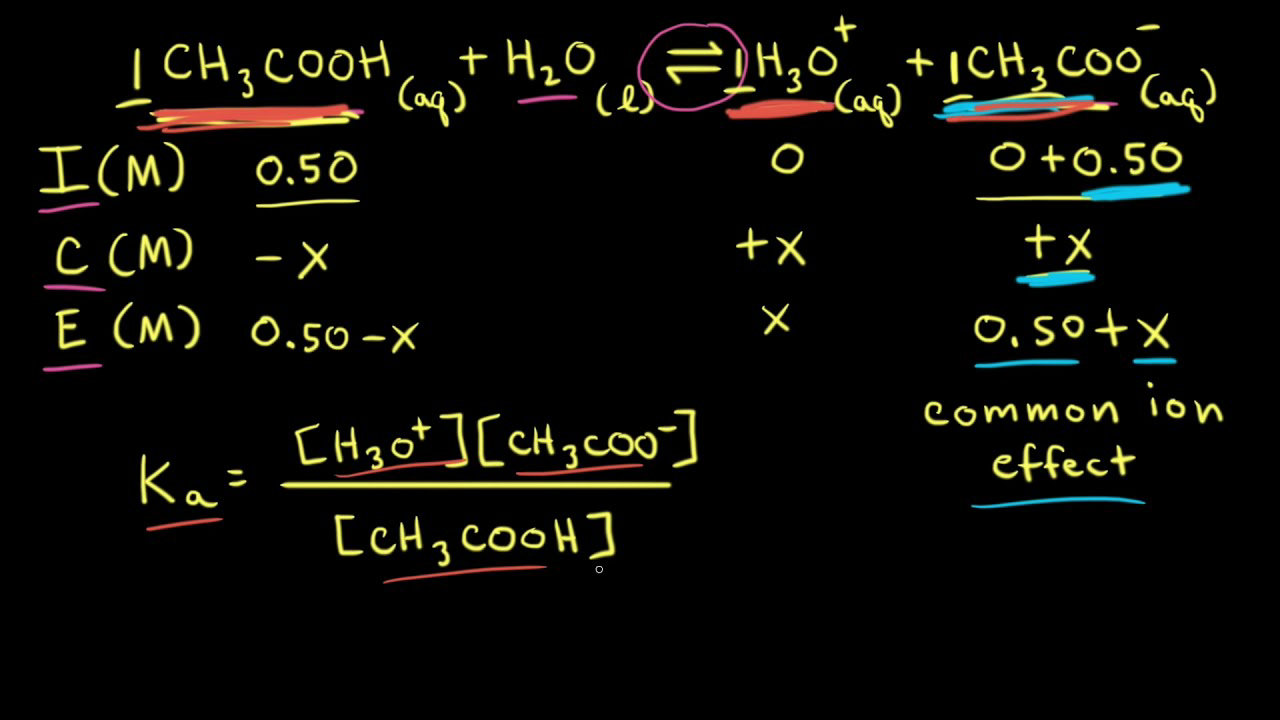
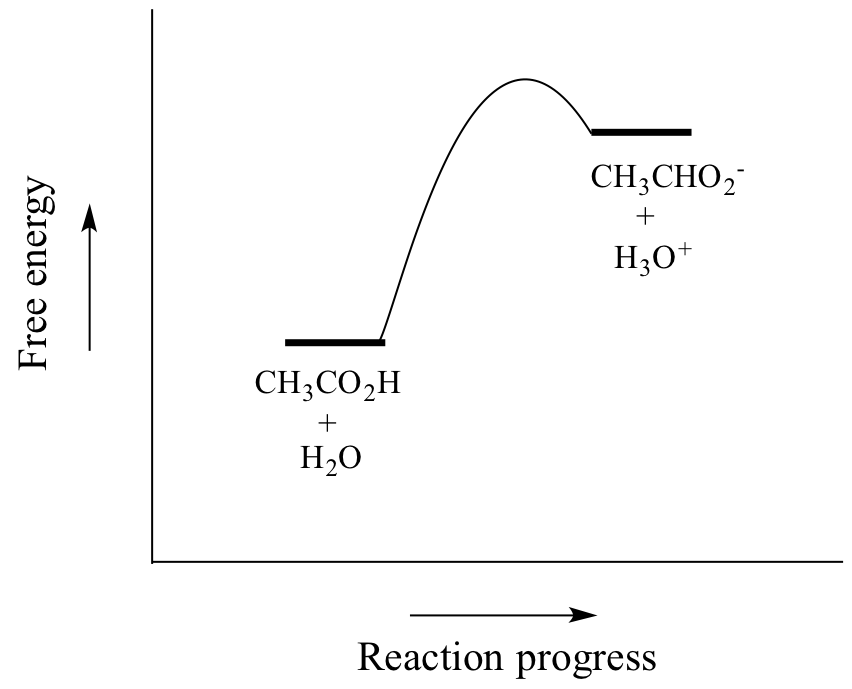
Worked example: Calculating the pH after a weak acid–strong base reaction (excess acid) (video) | Khan Academy

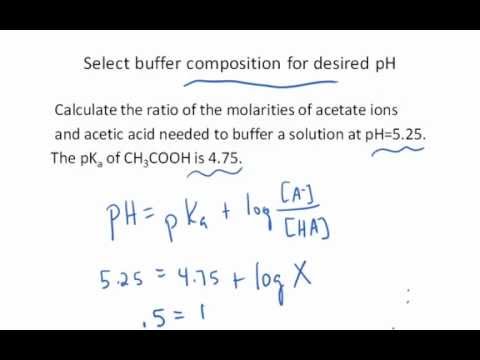
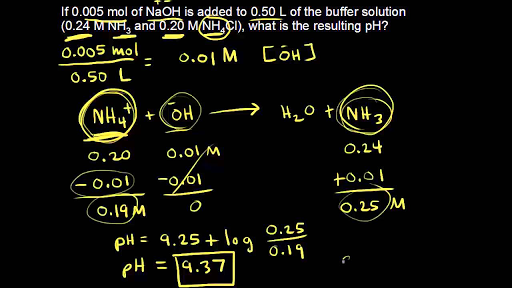
Determining the acid and conjugate base concentrations using the Henderson-Hasselbalch equation - YouTube

How to Calculate Analyte Concentration Using the Equivalence Point in an Acid-base Titration | Chemistry | Study.com